Whatsapp Contact Us +971 56 462 3659
Product
Product Center
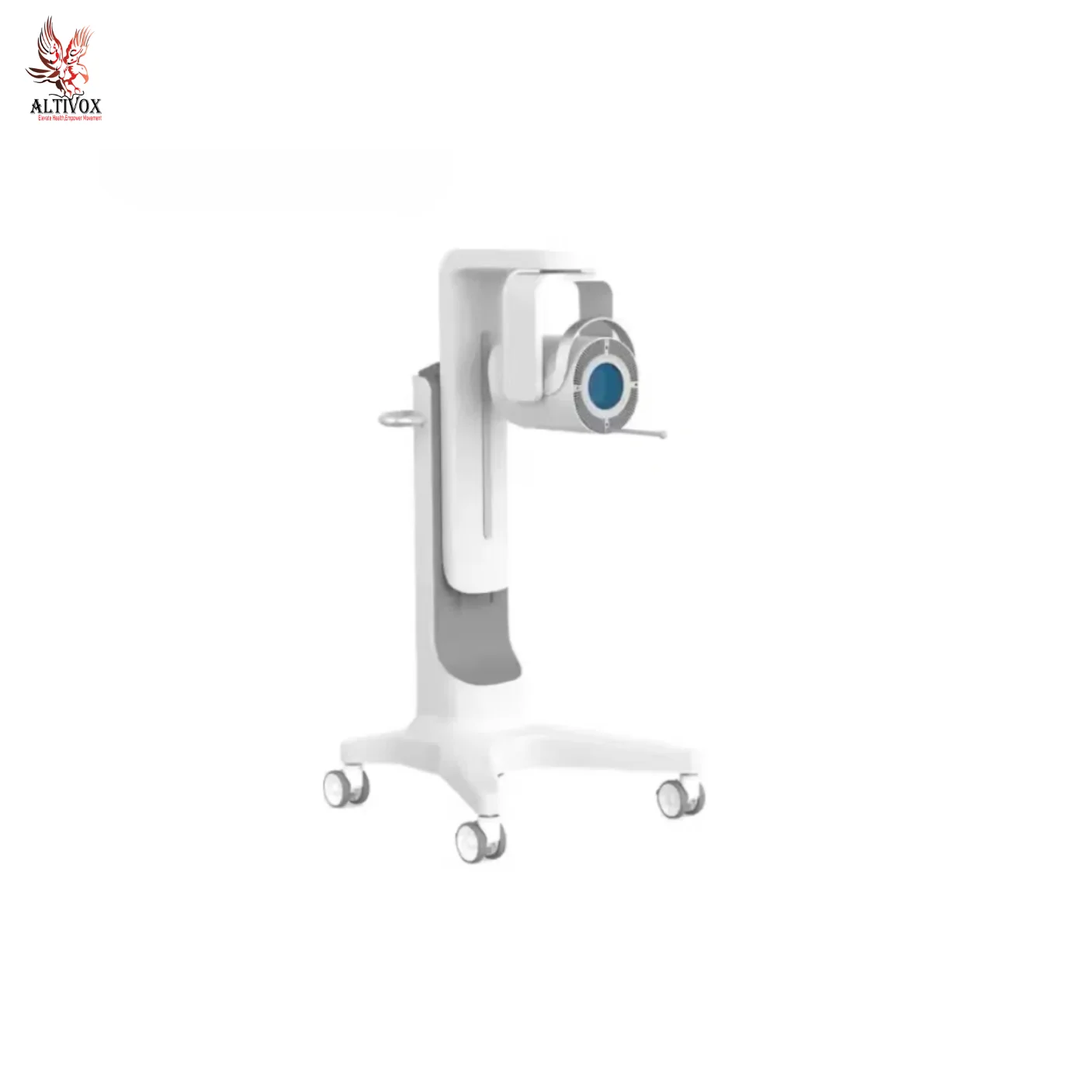
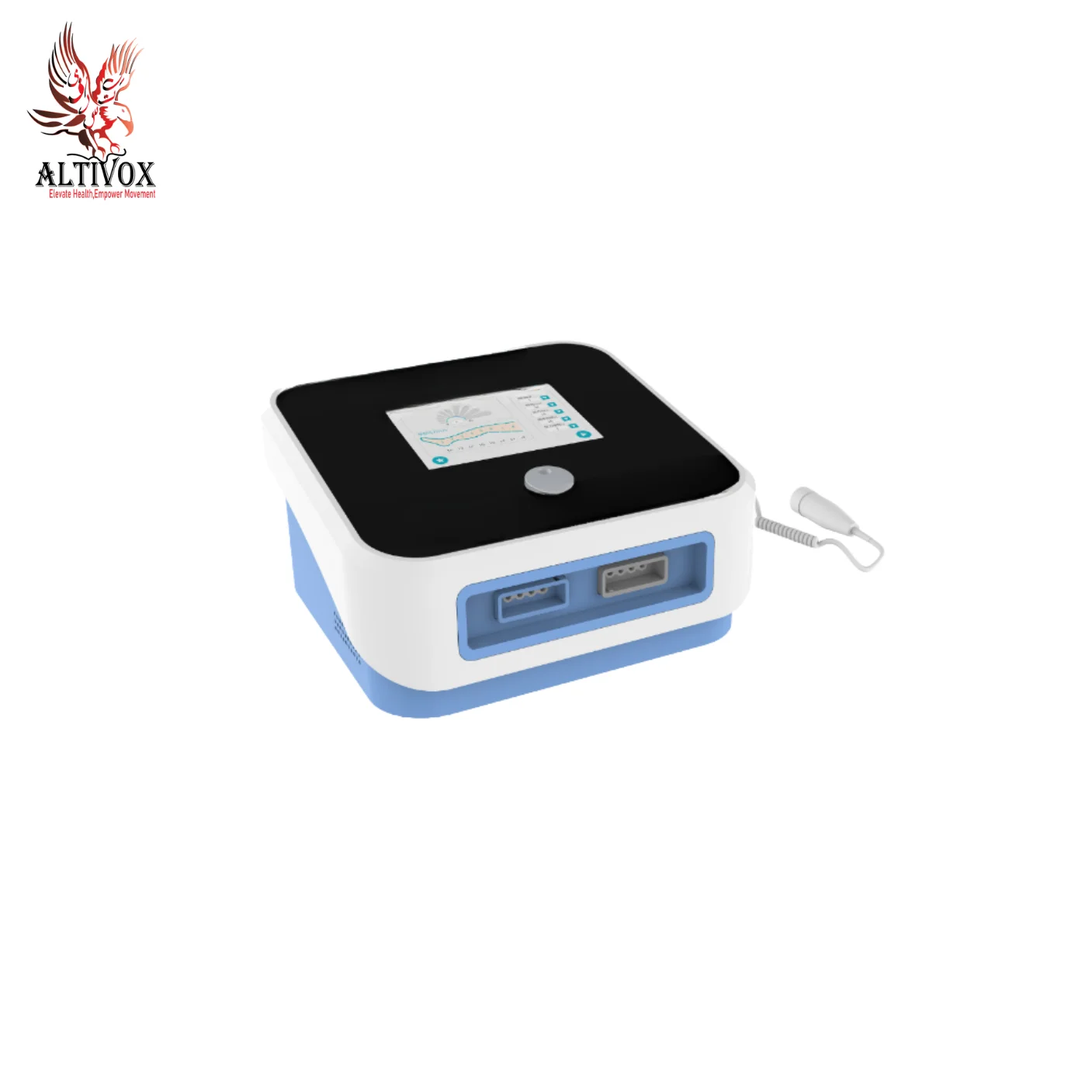
Extracorporeal Shock Wave Therapy Device AV-K-SHOCKMASTER-500
Model Number: XY-K-SHOCKMASTER-500
Certificate: ISO 13485, CE
Function: Reduce Pain
Dimensions: 500mm*435mm*1100mm
Voltage: 220V/50 Hz
Material: ABS
Instrument Classification: Class II
Extracorporeal shock wave therapy apparatus is produced by the compressor air pulse sound waves into precise ballistic burst that through physics conduction medium (such as air, liquid, etc.) acting on the body,produce biological effect, is the sudden release of energy caused by high pressure wave, with a higher pressure instantly and the characteristics of high speed transmission. Through the therapeutic probe .Positioning and moving can loosen the adhesions and dredge the tissues in a wide range of pain tissues.
Advantages
- New luxury appearance design, fashion and generous; Proll-type design, easy to move.
- Dual storage lockers: can store the therapeutic conductors and other sundries separately; Imported compressor, silent work,treatment of environmental safety,Quiet and comfortable; HD touch screen, easy to operate.
- Built-in 200 treatment prescriptions, additional treatment prescriptions can be added; Equipped with exquisite prescription manual and portable prescription desk calendar, you can choose treatment prescription according to the patient’s disease position; With voice broadcast function; With automatic pistol identification and detection function.
Scope of Application
It is suitable for the adjuvant treatment of pain.
Contraindications
- Pregnant or lactating women;
- Allergic constitution;
- The use of skin damage or skin disease;
- Patients with primary diseases of the heart, liver and kidney or hematopoietic system, patients with mental diseases, patients with built-in cardiac pacemakers and patients with implanted metal medical instruments.
Side Effects
- Edema, redness, hematoma.
- Bleeding points, pain.
- Skin damage after treatment.
These symptoms of the above side effects will automatically resolve after 5 to 10 days; Stop treatment if not eliminated.
Product Parameters
|
Product Name
|
Extracorporeal Shockwave Therapy Device
|
|
Rated Input Power
|
950VA
|
|
Impact Frequency
|
1Hz ~ 25Hz
|
|
Number of Shocks
|
100 ~ 9900 times
|
|
Working Pressure
|
1×10²kPa ~ 5.5×10²kPa
|
|
Size
|
500mm×435mm×1100mm
|
|
Impact Pistol Size
|
195mm×38mm×80mm
|
|
Rubber Gas Pipe
|
2400mm
|
|
Compressor
|
600kPa
|
|
Energy Density
|
0.15 mJ/0.6mJ/0.4mJ/0.85mJ/1.2mJ/7mJ/mm2
|
|
Penetration Depth
|
13mm/12mm /11mm /9mm ,/8mm
|
|
Pulse Width
|
380us/200us/190us , F15:180us , A6:320us
|
|
Prescription
|
200
|
|
Working Noise
|
Not Greater Than 70dB
|