Whatsapp Contact Us +971 56 462 3659
Product
Product Center
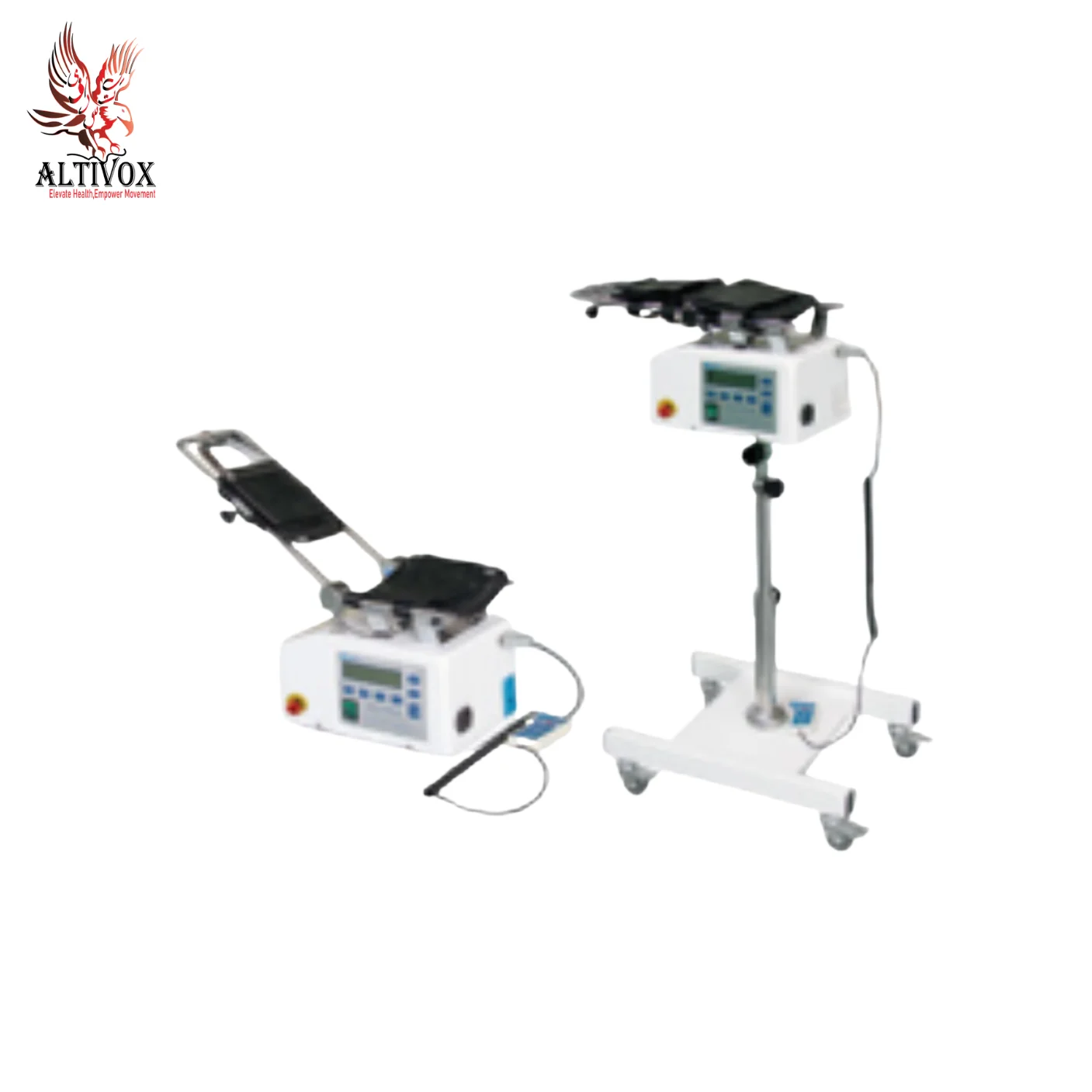
Recumbent Cross Trainer XY-SZLD-IA
Model Number: XY-SZLD-IA
Certificate: ISO 13485, CE
Equipment Size: 1520mm Width: 710mm Height: 1090mm Tolerance ± 5%
Step Frequency Range: 0-250 Steps/Minute
Resistance Adjustment: 10 Grades Resistance
Type: Rehabilitation Physiotherapy Equipment
The Limb Linkage Rehabilitation Training Instrument is a self-developed product based on the company’s original CPM product,drawing on advanced technology from similar foreign products. This product has made improvements in mechanical structure design and raw material use, particularly in electronic circuitry and software programming, resulting in an easy-to-use control interface. When the arm or foot begins to move, the display screen automatically shows relevant data. After four minutes of inactivity, the display will turn off automatically. The displayed information regarding patient treatment is easily readable.
These enhancements serve to further improve the rationality of the product structure, user convenience, and overall product reliability.
This training instrument utilizes purposeful exercise training to promote patient rehabilitation by engaging muscles throughout the body through a combination of sitting position and stepping exercises. Patients can utilize their healthy limb to drive movement on their affected side for active training while also engaging passive training with their affected limb. The driving force for movement on the affected side comes from the patient’s own healthy side movement, ensuring high safety standards are met.
Main Structure
The four-limb linkage rehabilitation training instrument is composed of a display screen, a resistance level adjustment handle, a seat (moving rod back and forth, moving rod left and right), a handle, a foot pedal, a bottom fulcrum, and a frame.
Scope of Application
It is suitable for rehabilitation training of limb muscle strength decline.
Contraindications
- Cerebrovascular accident sequelae can not cooperate with treatment;
- Patients with serious primary diseases such as cardiovascular, liver, kidney and hematopoietic system;
- Patients with severe osteoporosis or extreme weakness;
- Patients with untreated or untreated fractures requiring immobilization or lower limb trauma, bleeding and skin diseases;
- Patients with built-in cardiac pacemakers and other metal medical devices implanted in the body;
- Pregnant, lactating and pregnant women;
- Mental abnormality can not cooperate.
Product Parameters
| Product Name | Limb Linkage Rehabilitation Training Instrument |
| Input | a.c.220±22V, 50HZ±1HZ |
| Maximum Load Capacity | 2000N |
| Dimensions | 1655mm × 750mm × 1200mm |
| Seat Front and Rear | 0 ~ 325mm |
| Handle Length Adjustment | 0 ~ 400mm |
| Angle of Motion | 31° |
| Resistance System | 0 ~ 20Nm |
| Motor Output | Divided into High, Middle, Low 3 |
| Service Life | 8 Years |